SurgVision is a high-tech company
We develop pioneering solutions
for fluorescence guided surgical and interventional oncology
EXPLORER AIR® II
a fluorescence imaging system for open surgical procedures
Innovative products
imaging systems and imaging agents are in the development pipeline
SurgVision
is part of the Bracco Group since October 2017
Fluorescence Guided Surgical and Interventional Oncology
The Global Challenge Currently, the identification of tumours during surgery or interventional endoscopy relies on visual inspection and palpation. As tumour tissue is sometimes difficult to distinguish from healthy tissue surgical resection of tumour is incomplete in up to 40% of procedures1. This has medical as well as financial implications, impairing patient treatment outcomes.
Our Response A development pipeline combining highly sensitive imaging systems with tumour targeted imaging agents to visualize tumours during surgical or interventional procedures in real-time, enabling a more sensitive and accurate detection of the tumour1.
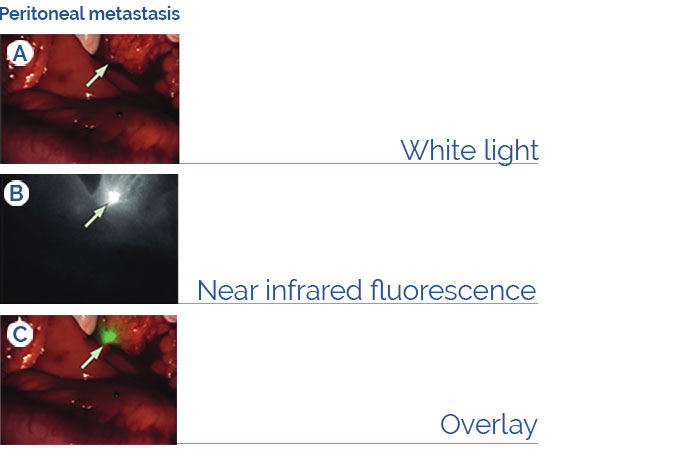
Fluorescence Guided Surgical Oncology
The imaging agent BEVA800TM labels cancer lesions during laparotomy in patients diagnosed with peritoneal carcinomatosis of colorectal origin employing the
EXPLORER AIR prototype (Harlaar et al. (2016) Lancet Gastroenterol. 1:283-90).
Disclaimer: BEVA800TM is under investigational use and not commercially available.
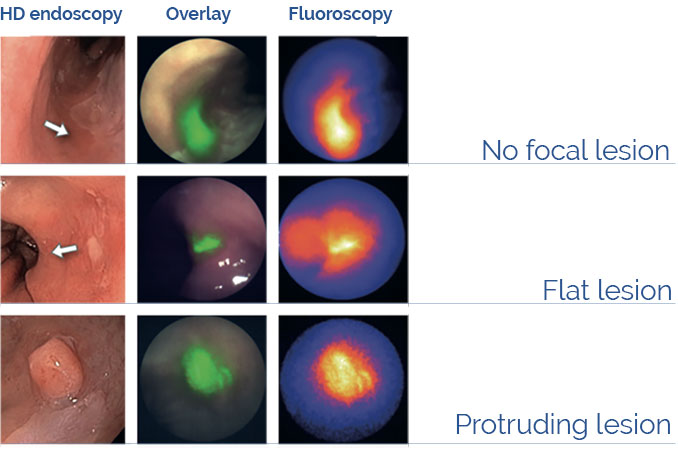
Fluorescence Guided Interventional Oncology
The imaging agent BEVA800TM labels cancer lesions in patients diagnosed with Barrett’s Esophagus employing the EXPLORER FLUOSCOPE prototype (Nagengast et al. (2019) Gut 86:7-10).
Disclaimer: BEVA800TM and EXPLORER FLUOSCOPE are under investigational use and not commercially available
1 Lamberts et al. (2016) Clin. Cancer Res.; Koller et al. (2018) Nat. Comm.; Harlaar et al. (2016) Lancet Hepatol.; Nagengast et al. (2017) Gut; Tjalma et al. (2020) Gut; Hartmans et al. (2018) Theranostics
EXPLORER AIR® II
The EXPLORER AIR® II is an intra-operative imaging system to visualize fluorescence in the near infra-red range in vivo. It has been designed to combine highest sensitivity with reliable imaging fidelity and convenience of use.
The system allows real-time imaging during surgery and its prototype has been tested by academic centres in a variety of indications 2.
The EXPLORER AIR® II is cleared by the US FDA for ICG based perfusion imaging as well as in conjunction with pafolacianine. It is CE marked for perfusion imaging in the EU.
As required by 2017/745 Annex I section 23.1 please find here the IFU of EXPLORER AIR® II. The file is password protected. If you are authorized, please send an Email to info@surgvision.com to receive the password.
2 Lamberts et al. (2016); Koller et al. (2018) Nat. Comm.; Harlaar et al. (2016) Lancet Hepatol.
Pipeline
high-performance technology
SurgVision develops a complete portfolio based on its proprietary high-performance technology to address the needs of next generation oncological intra-operative imaging.
High sensitive NIR Imaging System for open surgical oncology procedures
Clinical Application
Ovarian cancer, head & neck cancer, breast cancer, perfusion imaging, and others.
Availability
Cleared in the US for ICG based perfusion imaging
Cleared in the US in conjunction with pafolacianine
CE marked in the EU for perfusion imaging
Unique NIR imaging system for interventional oncology procedures
Clinical Application
Barrett's Esophagus, colorectal cancer, gastric cancer and others.
Availability
In development
High sensitive NIR Imaging System for minimal invasive surgical oncology procedures
Clinical Application
Lung cancer, prostate cancer, colorectal cancer and others.
Availability
In development
High sensitive NIR Imaging System agent for interventional oncology procedures
Clinical Application
Barrett's Esophagus, esophageal cancer, gastric cancer.
Availability
In development
Quality Management
SurgVision is ISO13485 certified and received
MDR clearance for EXPLORER AIR® II in 2022
History
Observing things under the right light, makes them clearer
First in human testing NIR imaging device in surgical oncology1
1 Van Dam et al. (2011) Nat. Med.
Foundation of SurgVision B.V.. Between 2013 and 2019: Clinical utility of the technology demonstrated in 6 clinical trials with > 160 pt in different open surgical and endoscopic settings2
2 Lamberts and al. (2016); Koller et al. (2018) Nat. Comm.; Harlaar et al. (2016) Lancet Hepatol.; Nagengast et al. (2017) Gut; Tjalma et al. (2020) Gut; Hartmans et al. (2018) Theranostics.
Comparison of Explorer Air prototype with other NIR imaging devices confirms outstanding performance 3
3 Dsouza et al. (2016) J. Biomed Opt.
Acquisition by Bracco
Start development of EXPLORER AIR® II
Conceptual design of Explorer Laparoscope
ISO 13485 / MDR compliance audit by notified body
EXPLORER AIR® II: 510(k) submission and CE class IIa request under MDR.
Explorer Fluoscope prototype: EMC testing passed
EXPLORER AIR® II: US 510(k) clearance for ICG based perfusion imaging
EXPLORER AIR® II: US 510(k) submission for expanded indication of use with pafolacianine
Get in Touch with Us
SurgVision GmbH
Kistlerhofstr. 70, Geb. 79
81379 Munich
Germany
info@surgvision.com
Privacy Policy | Terms of Use | Imprint
SurgVision is part of the Bracco Group